The Stakes Don’t Get Higher Than This: Racing Against the Virus to Help Moderna’s COVID-19 Vaccine Receive Emergency Use Authorization
by Penny Daniels
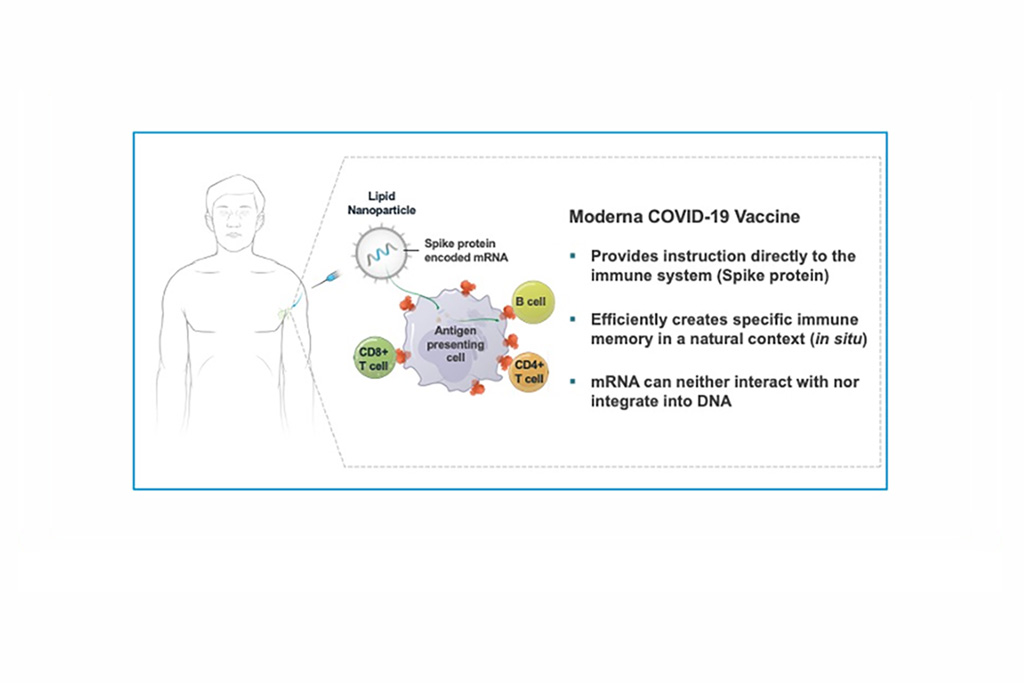
The Challenge
On New Year’s Eve, 2019, the world first heard about a COVID-19 coronavirus outbreak that would become the worst pandemic since the 1918 flu. Moderna, a biotech company developing mRNA medicines and vaccines, immediately began work on a vaccine against the SARS-CoV-2 virus. By mid-summer Moderna had started a Phase 3 clinical study in the U.S. and soon thereafter partnered with 3D Communications to prepare for the Food and Drug Administration’s (FDA) Vaccines and Related Biological Products Advisory Committee (VRBPAC) on the backdrop of a daily worsening pandemic. Our immediate and critical task: achieving a positive recommendation from the FDA’s VRBPAC for Emergency Use Authorization (EUA). To do this, we had to compress a typical six- to twelve-month preparation process into four to six weeks.
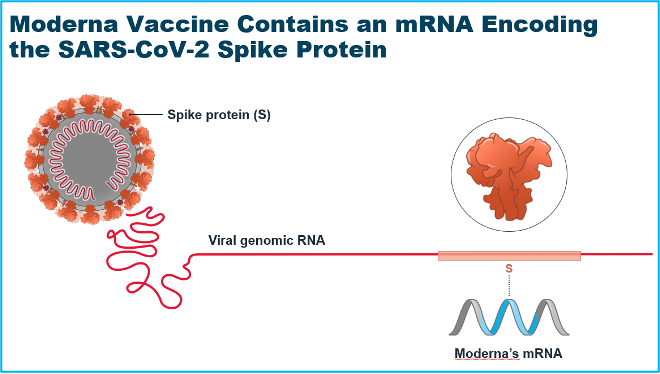
The Approach
With Moderna’s team working night and day to develop the EUA submission and the clock ticking, we had to develop a “shell” of the briefing book, presentation, and hundreds of Q&A slides, and later populate the slides with the Phase 3 data. We pride ourselves on our proven preparation process and our ability to be flexible and nimble when speed is critical – and never was it more so. 3D and Moderna worked as a cohesive team through weekends, nights, and over the Thanksgiving holiday with a clear mission – spotlight the vaccine efficacy, characterize the safety and treatment reactions and reassure the VRBPAC that the science was solid, and the public could be confident in the new vaccine.
Tal Zaks, Moderna’s Chief Medical Officer, said “The bar was high: the time pressure was unlike anything we’ve ever lived through, it’s the first-ever FDA Advisory Meeting for our company, and my Moderna team was simultaneously analyzing the data, preparing for VRBPAC, and preparing for parallel global regulatory submissions. We knew we needed professional help in preparing for VRBPAC. Everyone was impressed with 3D’s knowledge, work ethic, and technology. It was a pleasure to work with them. They really integrated with our team.”
The Results
At the VRBPAC, Moderna’s team was, in a word, spectacular. The data experts were fully prepared to address the committee’s questions and the moderator, presenters, and responders were honest, transparent, credible, and concise. To achieve this in such a short timeline was unprecedented.
On December 17, 2020 – a day when the CDC reported the death toll from COVID-19 in the U.S. had reached 306,427, 3,435 in the prior week alone – the VRPAC voted 20-0 with one abstention in favor of EUA. The FDA moved quickly, authorizing emergency use the very next day. It was a triumph for the country and the world, and we were honored and thrilled to be a part of it.
The Takeaway
The 3D ACT® Process, a committed and hard-working client, and lockstep collaboration works – even in the most condensed timeline. Everyone put their heads down, rolled up their sleeves, and worked against the clock and it paid off. The team presented the data and answered questions with depth and veracity, flawlessly transitioning between moderator and SMEs, and the vote was a clear cut 20:0 (with one abstention).