What You Need to Know About Application Orientation Meetings (Part 2): How to Prepare for Success
by Penny Daniels & Loren Reeside

An application orientation meeting, or AOM, is an optional meeting that the FDA or sponsors may request. These meetings involve a formal presentation by the sponsor, summarizing findings from the development program to support the marketing application.
The FDA review team will typically request an AOM within 45 days of submission, often leaving sponsors with little time to prepare.
Nevertheless, we still recommend that sponsors accept the meeting, as it is one of their first real opportunities to review data in detail with the agency.
In this follow-up article, Loren and I discuss how sponsors should prepare for these meetings.
_________________________
Penny: Will you review for me and our audience – what is the purpose of an AOM meeting?
Loren: These meetings are an opportunity for the sponsor to walk through the submission with the FDA review team and to provide their perspective. For the FDA it’s an early opportunity to hear the sponsor’s point of view and to ask any questions they may already have in their review of the submission.
Penny: If a sponsor agrees to an application orientation meeting with the FDA – what’s first? How should the sponsor go about preparing?
Loren: Similar to the way we advise sponsors to prepare for an FDA advisory committee meeting, we recommend they start by developing a timeline, working backward from the AOM meeting date. The FDA typically requests that a sponsor submit their presentation slides two to four business days before the meeting. Once a meeting date is set, estimate the date the slides will be due, if the FDA doesn’t provide those details. Then, schedule time for two to three internal team rehearsals prior to this date. The rehearsals give the team the opportunity to hear the presentation cohesively and to provide each other with honest feedback on each section. Each rehearsal should also incorporate a session to practice questions and answers, or Q&A. Designate a few days in between rehearsals for presenters to update their presentations based on feedback, and to prepare for Q&A. Now, working backward from the first rehearsal, sponsors will need to make time to identify presenters and prepare an initial draft of their presentation. Below is a visual representation of a typical timeline.
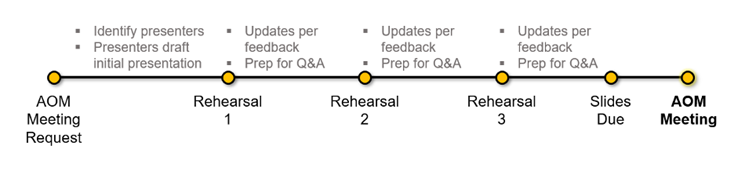
Penny: What type of content do these presentations generally cover?
Loren: Again, like an FDA advisory committee meeting, the primary focus should be the benefit-risk profile of the product, so components such as efficacy and safety, should be included to support that. However, that is really where the similarities with an advisory committee meeting end. For one, the audience is the FDA review team, not a panel of clinicians. Second, the review team has the entire submission, which includes much more data and details than an advisory committee briefing book, so the presentation should reflect that.
In addition, the FDA may propose a list of topics they would like discussed and sponsors should also address any additional issues that are uniquely relevant to their specific application. Remember, however, that this meeting is generally shorter than an advisory committee meeting; the presentation should be 40 minutes or less.
Penny: What does the general flow of the presentation look like?
Loren: We generally recommend starting with an introduction to an overview of the product, which may include the mechanism of action, a brief description of the unmet need/disease background, a summary of the benefit-risk message, and an agenda for the presentation. Next, the primary efficacy and safety clinical data → followed by brief pharmacology, CMC, and nonclinical data → and then, concluded with a benefit-risk summation. While the elements may vary from an advisory committee meeting, the flow still generally follows best practices for presentations: i.e., tell them what you’re going to tell them, then tell them (providing data to support the messages), and then conclude by telling them what you told them.
Penny: With this being one of the first meetings with the FDA review team in the regulatory pathway to approval, how much time is spent on messaging? It seems it would be pretty important to identify those key messages upfront.
Loren: Absolutely. Sponsors want to make sure the messages are clear, compelling, and consistent throughout the review process – from the AOM to the briefing document submission and finally, at the FDA advisory committee meeting. The team should message around why the product is needed (for example: a rare disease with no other treatment options) and why the efficacy outweighs any risks that may be associated with the product. Individual presenters should be thinking of any supporting key messages they want to convey in their sections. Presenters should then script their presentations with these key messages in mind.
Penny: Earlier you mentioned feedback after each rehearsal. What advice can you provide on what the team should be focusing on during these practice sessions?
Loren: The first thing to track (and conveniently, the easiest) is the overall time of the presentation. Staying on time is critically important. If the FDA requests a 35-40 minute presentation, the sponsor should deliver exactly that. Furthermore, capturing the time will serve as a benchmark to let the team know whether they need to cut content or whether they’re on track and can focus primarily on refinement. There is always a lot more data that goes into submission than there is time to present, so determining what to present, in the given time, is always a challenge. Tracking time can help force a team to make tough decisions about what to include and exclude. After a time, the team should focus on whether their key messages came across and if so, were they strong enough? What information should perhaps be saved for Q&A? Even if the presentation meets the allotted time, are there data being shown that aren’t adding to the presentation, or may even be obscuring the messages? How were the overall organization and presentation flow? Also, I should note because we often get this question – while script drives time, speaking faster to make time is not an option! If a presenter speaks too quickly, people cannot absorb the information. Presenters should speak clearly and confidently at a normal conversational pace. If that isn’t possible, the script needs to be cut. Say less, communicate more – that’s our motto.
Penny: Let’s talk Q&A – following the presentation the review team has 15-20 minutes to ask questions. How should a sponsor prepare for this?
Loren: The sponsor should identify the top questions that they think the review team is most likely to ask. These questions might involve issues or concerns already received from FDA along the pathway to submission. They may also be questions that are extremely difficult, the type that would “keep you up at night.” If there are questions the sponsor hopes they won’t be asked – they need to prepare for those. Then, while presenters are working on their talks and updating them, other team members can develop answers and supporting slides. The team should also think about the appropriate responder for each question. Each responder must review the developed responses from their team to ensure they’re accurate and credible. Practicing Q&A will drive further refinement of both responses and backup slides.
Of note, the FDA has typically requested that backup slides be submitted with the presentation, so any slides the team may want to show for Q&A will need to be finalized by the same deadline.
Penny: You recommended that sponsors always accept the invitation for an AOM? Where is the upside? So far it just sounds like a lot of work.
Loren: This is one of the sponsor’s earliest opportunities to make the case for why their product should be approved. In addition, a sponsor should generally demonstrate a willingness to work with the FDA, especially when the request comes from a desire to better understand the submission. Furthermore, as part of the Q&A portion of the meeting, a sponsor can get a preview of some of the hurdles they may face along the pathway to approval.
The other potential benefit is the opportunity to ask the FDA if it’s currently planning an advisory committee meeting. While there is no guarantee the Agency will have an answer, some of our clients have been able to gather that valuable information as early as their AOM. Depending on the response, this may give sponsors a head-start on preparing for an FDA advisory committee meeting or, even better, an opportunity to breathe a sigh of relief.
The good news is the 3D team is here to support sponsors in preparing for all regulatory meetings, with experience supporting many clients in preparing for an AOM.